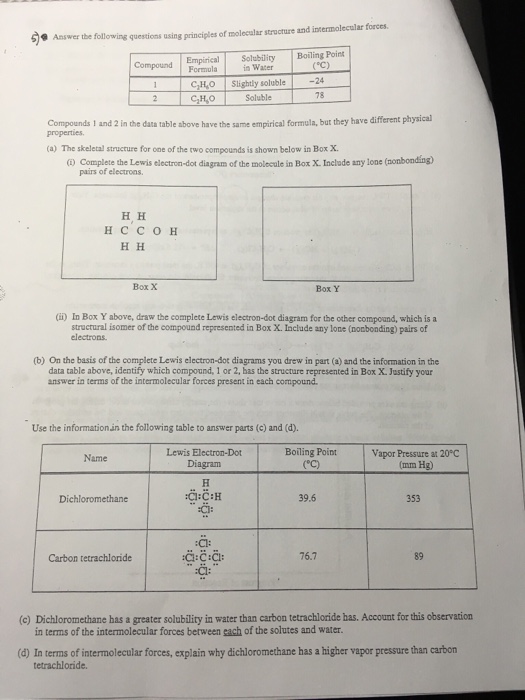
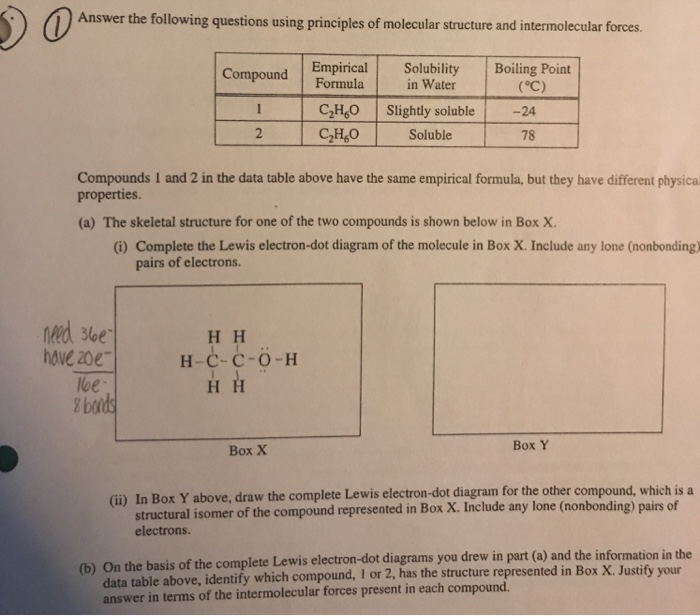
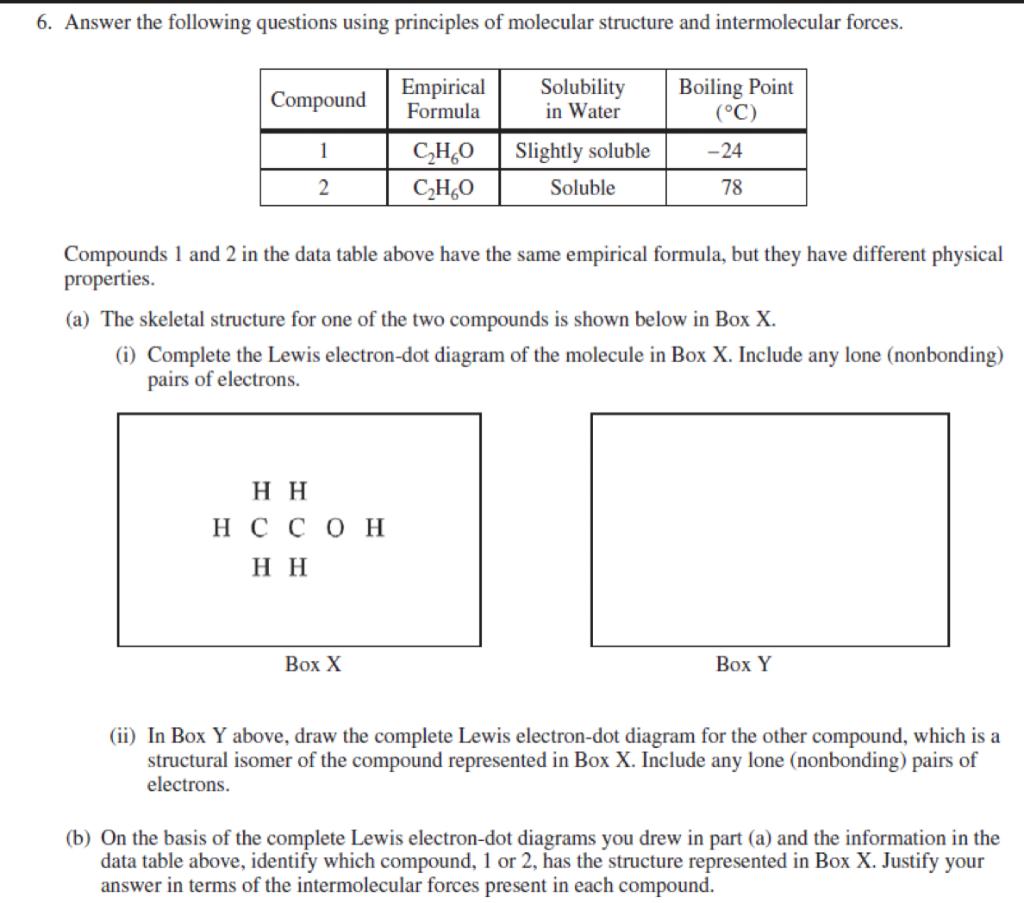
The complete Lewis electron-dot diagram for methanamide is shown below. Draw circles around each oxygen atom in the dioxygen Lewis diagram above to show the octet rule.
Solved Answer The Following Questions Using Principle Of Chegg Com
All of these obey the rule that there are eight electrons around each atom.
. Draw a skeleton joining the atoms by single bonds. Y 2p z l 1 x y z n 2 This is an accurate representation of a 2p x orbital. The circles do not need to be drawn perfectly circular but they do need to enclose the correct electrons.
8 6 7 50. In the spaces below draw a Lewis dot diagram showing the presence of all electrons in the molecule or ion. This is a common picture of a p x orbital This simplifi ed p x orbital is often useful.
The structure of this molecule has a geometry around the carbon atom similar to the geometry around carbon in methanamide. Y x z y x z Use these axes. The valence electrons are written as dots surrounding the symbol for the element.
See how in this case each oxygen atom shares two. 2 points are earned for a correct Lewis electron-dot diagram for formic acid. Consider a molecule with the formula CH2O2.
You need to use a pen or even a dotting Device to obtain this final result. You may need a little something to produce modest dots with. Estimate the observed ingle.
Electron Geometry Molecular shape CBr4 NBr Number of nonbonding pairs. Up to 24 cash back halogens and finds the formula CIF3. BEthyl methanoate CH3CH2OCHO is synthesized in the laboratory from ethanol C2H5OH and methanoic acid HCOOH as represented by the following equation.
HCO 3 ion that is are consistent with the given information. Draw Lewis symbols of the individual atoms in the molecule. 8 2 7 22XeF 6.
In the box provided below draw the complete Lewis electron-dot diagram for the molecule. One dot is place on each side first and when all four positions are filled the remaining dots are paired with one of the first set of dots with a. Total number of electron groups.
The steps to draw the Lewis structures of various types of compounds are given below. Four water molecules are represented in the box below. Then top rated it off with a yellow dot at the center.
We can combine all three p orbitals in a three dimensional display. Use this box to draw a p z orbital. In this case we can condense the last few steps since not all of them apply.
This nail art is great for the spring and summer. In the molecule angle at is not 180. Draw Lewis structures that show how electron pairs move and bond from and breaks in this reaction and identify the Lewis acid and Lewis base.
Is a liquid at 25 degree C. In the box below draw the complete Lewis electron dot diagram of a methanoic acid molecule. There may be more than one possible structure but you should draw only one configuration but draw it clearly placing your final answer in the box.
A Lewis structure is a graphic representation of the electron distribution around atoms. Lewis Line Like fl uorine in difl uorine each oxygen atom in dioxygen is isoelectronic to neon. B On the basis of the Lewis electron-dot diagram that you drew in part a predict the molecular geometry of the IF 3 molecule.
The student adds two drops of 30 M HNO. Lewis Structure of oxygen left rmO_rm2 right Oxygen belongs to group 16 of the Periodic Table. Number of bonding groups Total number of electron groups.
One point is earned for a correct Lewis diagram can be done with dots or lines. Bring the atoms together in a way that places eight electrons around each atom or two electrons for H hydrogen wherever possible. C2H5OHl HCOOHl CH3CH2OCHOl H2Ol i.
Each 33 Given the reaction. 2 pts BONUS Questions 1 pt. The reason for learning to draw Lewis structures is to predict the number and type of bonds that may be formed around an atom.
View the full answer. Each pair of shared electrons is a covalent bond which can be represented by a dash. In the molecule angle v is no.
Number of nonbonding pairs. Calculate the number of valence electronsXeF 2. Include any charges or partial charges 1 pt 32 Explain in terms of electronegativity why an H-F bond is expected to be more polar than an H-I bond.
Make 5 dots so that they connect. Draw ONE dashed line ---- to indicate a possible location of a hydrogen bond between a water molecule and the urea molecule. Explain why in terms of electron domains VSEPR model.
Number of bonding groups. In the box provided below draw the complete Lewis electron-dot diagram for the molecule. HC 2H 3O 2 and NaC 2H 3O 2.
I point is earned for a central Cl atom surrounded by three bonding pairs with F atoms and two nonbonding lone pairs of electrons. The solution is measured to be. Up to 24 cash back methanamide.
A Lewis structure also helps to make a prediction about the geometry of a molecule. Up to 24 cash back Lewis electron-dot diagrams for C02 and S02 are given above. In the box below draw a complete Lewis electron-dot diagram for a molecule of CIF3 See diagram above.
E In the box below draw the Lewis electron-dot structure for the compound formed from magnesium and chlorine. One of the oxygen atoms. Hence oxygen has 6 valence electrons.
Ater drawing the Lewis structure in the large box complete the table. F A student prepares a solution containing equimolar amounts of. A student determines that 539 grams of H.
A hand drawn version does not have to be exact. In the box below draw a Lewis electron-dot diagram or diagrams for the. We can draw the Lewis structure of any covalent molecule by following the six steps discussed earlier.
Steps to Draw Lewis Structure. A In the box provided below draw a complete Lewis electron-dot diagram for the IF 3 molecule. H2 Cl 2 2HCl.
In box y above draw the complete lewis. The number of dots equals the number of valence electrons in the atom. The molecular geometry and polarity of the two substances are A the same because the molecular formulas are similar B the same because C and S have similar electronegativity values C different because the Ione pair of electrons on the S atom make it the negative end of a dipole.
F atoms must have three nonbonding pairs each. 2 aq The dissolution of urea is represented by the equation above. Step 1 Calculating the total valence electrons.
1 point is earned for the correct skeletal structure for formic acid with the CO double bond ie containing all five bonding pairs. A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. These dots are arranged to the right and left and above and below the symbol with no more than two dots on.
2 s H. Lewis dot diagrams for elements are a handy way of picturing valence electrons and especially what electrons are available to be shared in covalent bonds.

Solved Open Ended Show All Work When Applicable 16 Answer Chegg Com
Solved Answer The Following Questions Using Principles Of Chegg Com

Pre Test Big Idea 2 Chapters 8 9 10 Pdf Free Download
Solved 6 Answer The Following Questions Using Principles Of Chegg Com
0 comments
Post a Comment